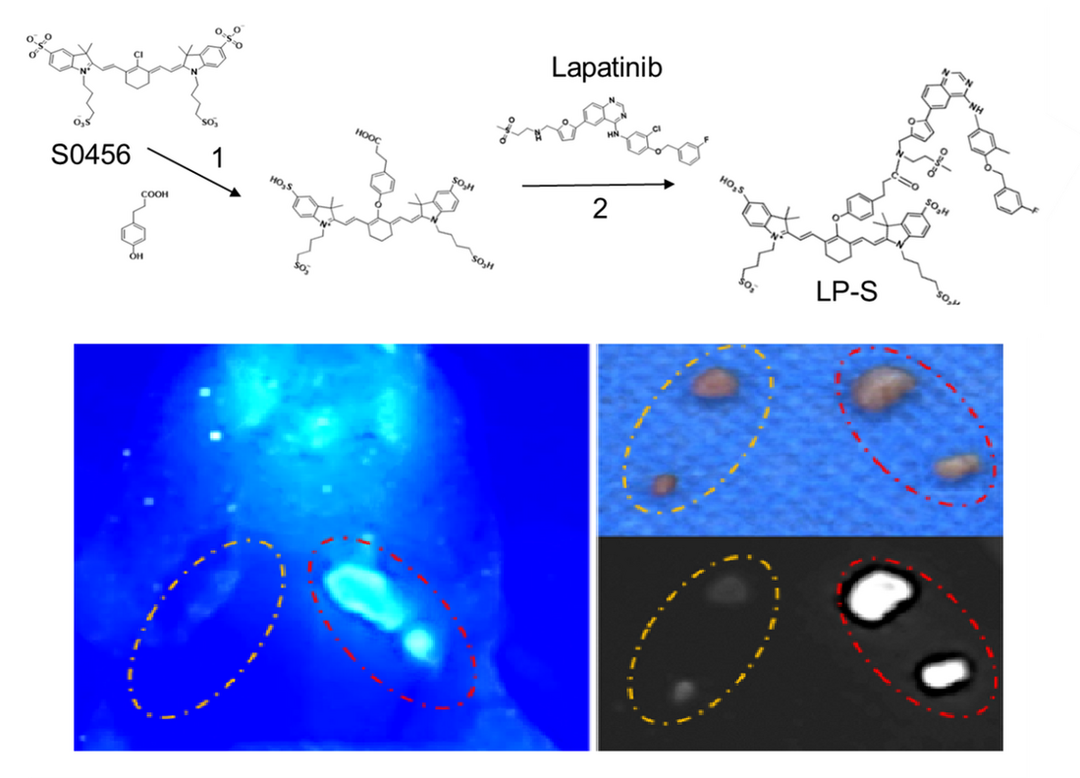
We've developed a promising new tool that could make oral cancer surgery more precise. Our team created a specialized fluorescent dye called LP-S that lights up metastatic lymph nodes, making them easier for surgeons to identify and remove.
Right now, surgeons rely on a standard dye called ICG, but it's not very specific - it highlights everything, making it hard to tell which lymph nodes actually contain cancer cells. Our new dye is much more precise, specifically binding to EGFR receptors, which are found in high concentrations on oral cancer cells.
When we tested LP-S in mouse models, the results were impressive. The dye produced clearer, longer-lasting fluorescence in cancerous lymph nodes compared to ICG, giving surgeons a much better view of what they're working with. This means they can more accurately remove the correct lymph nodes while leaving healthy tissue in place.
If this technology makes it through clinical testing, it could be a game-changer for oral cancer patients. Better lymph node mapping means more accurate cancer staging and ultimately better treatment outcomes.
Right now, surgeons rely on a standard dye called ICG, but it's not very specific - it highlights everything, making it hard to tell which lymph nodes actually contain cancer cells. Our new dye is much more precise, specifically binding to EGFR receptors, which are found in high concentrations on oral cancer cells.
When we tested LP-S in mouse models, the results were impressive. The dye produced clearer, longer-lasting fluorescence in cancerous lymph nodes compared to ICG, giving surgeons a much better view of what they're working with. This means they can more accurately remove the correct lymph nodes while leaving healthy tissue in place.
If this technology makes it through clinical testing, it could be a game-changer for oral cancer patients. Better lymph node mapping means more accurate cancer staging and ultimately better treatment outcomes.